A review on the use of glassy carbon in advanced technological applications
Abstract
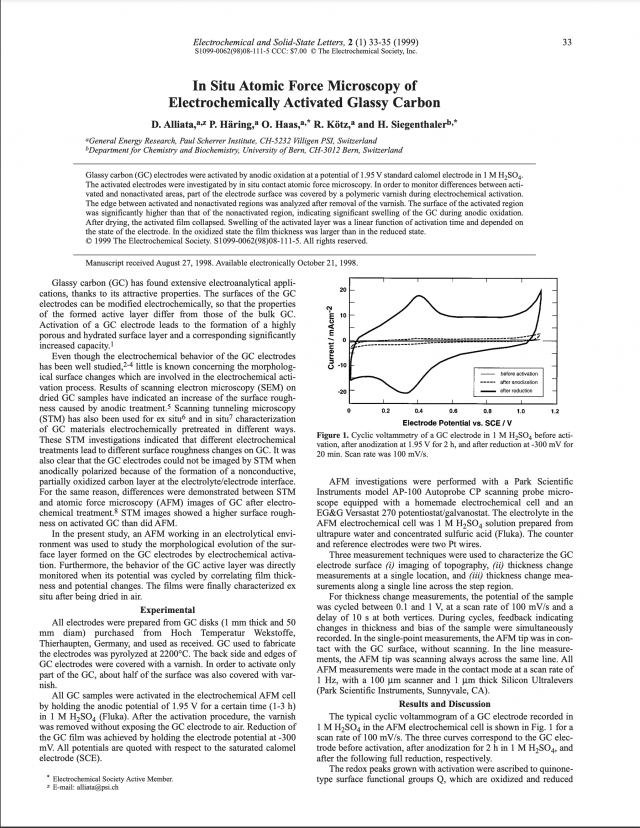
Recently, many studies have been conducted on the use of glassy carbon (GC) in advanced technological applications due to its excellent chemical, mechanical, electrical, and thermal properties, making possible fast advances in biomedical, pharmaceutical, electronic, and energy sectors. In this way, this review article reports the latest advances (2017–2021) in the scientific research on the use of GC in diverse technological applications, including scaffolds for tissue engineering, electrochemical sensors for molecular determination, energy storage systems, electrochemical devices for wastewater treatment, tools for precision molding, encapsulation of nuclear waste, and antistatic agent for antistatic packaging. A brief analysis of the number of published articles on the topic is presented, showing how the use of GC has evolved over the years in different technological sectors. The structure, the properties, the production of GC, and the promising areas for further research are also addressed, providing helpful information to foment scientific advances on the use of GC.